|
|
There’s a Way to Better Batteries. Produced Without Human Suffering and Environmental Destruction
Lithium-sulfur cells can deliver several times more energy in the same volume as lithium-ion ones. However, they had one fundamental problem – their short lifespan. It seems that this issue has been resolved. This could bring relief to millions of people and animals.
Who Wouldn’t Want to Charge Their Smartphone Only Once a Week?
Commonly used lithium-ion cells in most devices today have a capacity ranging from 150 to 260 watt-hours per kilogram (Wh/kg). There are also lithium-sulfur cells – and they have a much larger capacity, about 550 watt-hours per kilogram.
At the same weight, a lithium-sulfur battery lasts two to three times longer than a lithium-ion one. It is also about half as expensive because it uses much cheaper sulfur instead of relatively expensive cobalt found in lithium-ion batteries.
Lithium and sulfur are light enough elements that such cells are also very light – two and a half times lighter than the lightest lithium-ion ones.
The weight of the latter may not be a problem in the case of a smartphone (although everyone would prefer lighter rather than heavier), but light batteries would be very useful in electric cars, which are much heavier than combustion engine cars today. The average electric car battery weighs from 450 kilograms upwards (sometimes reaching nearly a ton), significantly increasing the vehicle’s mass.
The Problem with Chains (of Sulfur)
Since lithium-sulfur cells have so many advantages, why aren’t they everywhere yet? Unfortunately, they suffer from a fundamental flaw – a much shorter lifespan.
Today’s commonly used lithium-ion cells last about 2,000 charging cycles. Lithium-sulfur cells have a much shorter life – about 1,000 charging cycles.
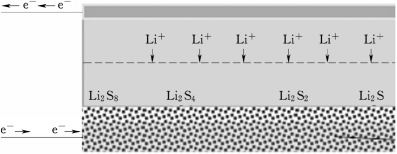
The source of this problem is the chains of lithium polysulfides that form during each charge, which simply clog such batteries. Many methods have been tried to increase the lifespan of such cells, but each had its drawbacks.
Engineers from Australia’s Monash University claim to have found a solution. They developed an anode design (in cells and batteries, this is the negative electrode) that significantly extends the life of lithium-sulfur batteries.
The anode in their cell is a thin layer of lithium covered with a special film with pores the size of millionths of a meter. As the inventors explain, lithium ions can freely penetrate through them, but larger sulfur ions cannot.
Is It Time for Lithium-Sulfur Cells?
Does this mean the long-awaited breakthrough and lithium-sulfur batteries will conquer the market? It’s wise to be cautious. This isn’t the first time scientists in laboratories have announced that they have managed to extend the life of such cells.
Lithium-sulfur cells are not a new invention (American Patent)
Working principle diagram of lithium–sulfur battery
S8+16e−+16Li+→8Li2S
The following is the reaction equation of a series of polysulfide ion:
S8→Li2S8→Li2S6→Li2S4→Li2S2→Li2S
However, the first lithium-sulfur cells with a lifespan of a thousand charging cycles were only constructed in the early 2000s. In 2017, it was possible to achieve a lifespan of 1500 cycles – still less than lithium-ion, but close. In 2022, researchers from Drexel University stated that by using one of the forms of sulfur (its allotropes), it was possible to achieve even 4000 charging cycles.
That’s twice as much as lithium-ion cells. Who wouldn’t want cheaper, lighter, more capacious, and more durable batteries… Yet such batteries are still not on the market. Why?
One of the reasons is the difficulty in developing technology that allows for the production of such cells on a mass scale. Just because something is successful in the laboratory doesn’t mean it’s easy to manufacture in a factory. Scaling up technology from a few prototypes to hundreds of thousands of units also takes quite a long time. They Also Waited a Long Time. And They Received a Nobel Prize
There is nothing particularly strange about this wait for progress. Lithium-ion batteries, which are so common today, also lay in the laboratories of chemists and engineers for a long time, but they were a bit luckier.
They also have their origins in the 1960s. A quarter of a century passed, and in the mid-1980s, it was discovered that their efficiency is greatly improved by petroleum coke (later replaced by graphite). Another decade passed when it was discovered that their durability is improved by ethylene carbonate (an electrolyte that is solid at room temperature).
Only then, at the end of the last century, did the revolution begin, which allowed powering smartphones and electric cars. For this, the creators of these batteries, John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino received the Nobel Prize in Chemistry in 2019.
If it becomes possible to start producing lithium-sulfur cells on an industrial scale, this revolution will accelerate. A charged battery in a smartphone will last three days (who knows, maybe even four). Electric vehicle batteries could be halved in weight and still achieve twice the range. It will be possible to store more solar and wind energy – which the world would very much need.
The environment and the people mining cobalt will also breathe a sigh of relief. In lithium-ion batteries, cobalt is essential. Most of it is mined in the Congo basin in Africa.
More Articles about this topic:
Our Article about Lithium Extraction
A Revolution, Unfortunately Not Bloodless
In the tropical forests of the Congo basin, Africa’s Amazon, live over a thousand species of birds, 700 species of fish, and 400 mammals. It is a unique habitat for elephants, hippos, mountain gorillas, and the largest population of chimpanzees.
Unfortunately for them, these lands are very rich in rare and valuable metals – including cobalt, essential for our batteries. Forests are cleared for mines, and animals lose their natural habitat.
The development of metal mining in the Congo basin has also led to bloody wars in Rwanda, Uganda, Angola, and the longest in the eastern part of the Democratic Republic of Congo, which has been ongoing since the 1990s and is called the “African World War.”
Congo Cobalt Production
The fighting between armies and guerrillas over access to deposits of valuable and rare metal ores has claimed (directly or due to famine) over 5 million fatalities. Another two million have been forced to leave their homes. Tens of thousands of refugees, including children, work in the mines. Some out of a lack of means to live, some at gunpoint.
This is too often the silently paid price for all modern electronics, especially those powered by batteries.
Lithium-sulfur cells could stop this violence and destruction because cobalt is unnecessary in them. The world would very much need them.
Sources:
- https://ourworldindata.org/
- https://oko.press/lepsze-baterie-litowo-siarkowe
- https://www.nobelprize.org/prizes/chemistry/2019/summary/
- https://www.sciencedirect.com/topics/engineering/lithium-sulfur-batteries