|
|
A groundbreaking study by the National University of Singapore (NUS) heralds a significant leap forward in sustainable hydrogen peroxide (H2O2) production, promising a new environmental and industrial efficiency era.
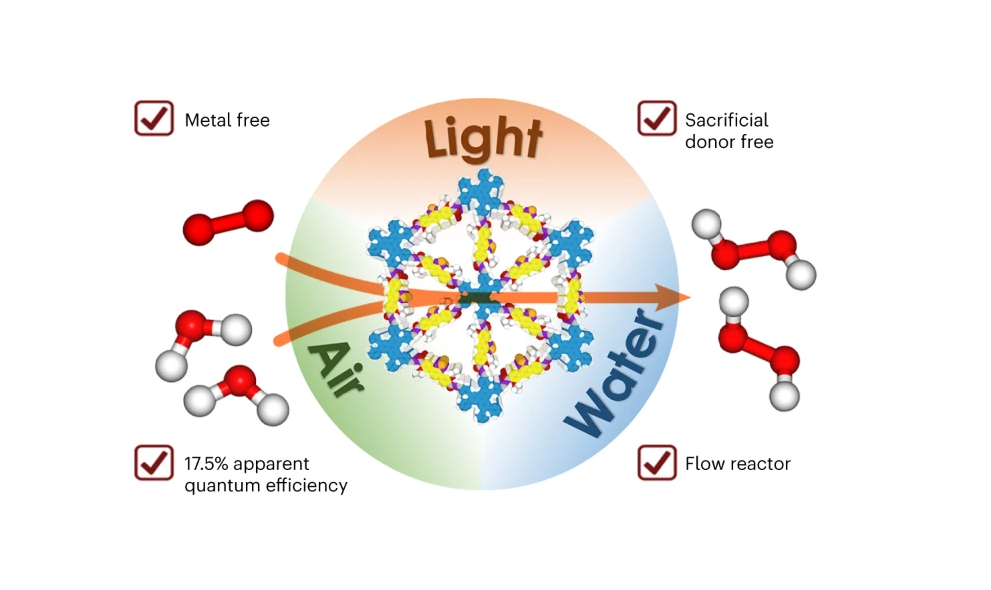
The research team, led by Professor Jiang Donglin of the NUS Department of Chemistry, has unveiled a novel method that leverages sunlight to synthesize H2O2 directly from water and air, employing a unique hexavalent covalent organic framework (COF).
The key to this innovative technique lies in the specially designed COFs that feature dense donor–acceptor lattices and engineered linkages, fostering a high rate of photo-induced charge generation and providing catalytically active sites. These hexavalent COFs are meticulously structured with microporous channels that ensure the rapid delivery of water and oxygen directly to the sites where H2O2 synthesis occurs, thus maximizing efficiency.
Demonstrating a quantum efficiency of 17.5 percent under visible light at 420 nm in batch reactors, this method not only sets a new benchmark in photocatalytic efficiency but also opens up exciting applications, including developing self-cleaning surfaces and disinfection treatments.
Traditional Production of Hydrogen Peroxide
Traditionally, hydrogen peroxide production has relied on a method known as the anthraquinone process. This technique accounts for an estimated 95% of the hydrogen peroxide manufactured worldwide. Its widespread adoption is due to its tried-and-tested nature, which has been refined over the years to meet global demand.
The anthraquinone process unfolds through four stages: hydrogenation, oxidation, extraction, and treatment of the working solution. Each stage is crucial in transforming raw materials into highly useful hydrogen peroxide.
- Hydrogenation: During this phase, anthraquinone—an organic compound—is combined with hydrogen. This step sets the stage for chemical reactions essential for creating hydrogen peroxide’s foundation.
- Oxidation: The previously treated anthraquinone is exposed to oxygen at this stage. This exposure triggers a critical reaction for forming the basic structure of hydrogen peroxide.
- Extraction: After oxidation, the mixture contains the desired product but not its pure form. Therefore, this step carefully separates hydrogen peroxide from the rest of the mixture. This phase is vital for ensuring that what moves forward is as close to pure hydrogen peroxide as possible.
- Treatment of the working solution: Once the hydrogen peroxide has been extracted, what remains needs to be recycled and prepared for reuse in another production cycle. This treatment ensures the process is as efficient and sustainable as possible within its existing framework.
Although it has been around for quite some time, the anthraquinone process has its share of problems.
First, the method is a hungry energy consumer, requiring a lot of power to run, which immediately raises red flags in today’s energy-conscious world. But that’s not the only issue. The process also leans heavily on the use of toxic solvents. These substances harm people and the planet, posing severe risks if they leak or are disposed of improperly.
Moreover, creating hydrogen peroxide this way demands the use of expensive noble-metal catalysts. These costly materials add another layer of complexity to the production process. And where there’s complexity, there are more chances for things to go wrong.
But perhaps one of the most pressing concerns with the anthraquinone process is the amount of waste it generates. The method is notorious for its side reactions—unintended chemical reactions that occur alongside the desired one. These reactions not only reduce the efficiency by consuming resources and producing unwanted byproducts but they also add to the environmental burden.
Implications of This New Sustainable Production of Hydrogen Peroxide
Hydrogen peroxide serves multiple purposes, functioning as a bleaching substance, disinfectant, oxidizing agent, and catalyst for initiating and crosslinking polymers. Its applications span numerous industries, including textiles, paper production, chemical manufacturing, military uses, electronics, food processing, pharmaceuticals, cosmetics, environmental conservation, and metallurgy.
As of 2018, worldwide usage of hydrogen peroxide was approximately 6.5 million metric tons, a figure that has been on a swift upward trajectory.
The development of a novel method for synthesizing hydrogen peroxide directly from water and air, using sunlight and a specially designed hexavalent covalent organic framework (COF), marks a significant milestone in sustainability and industrial efficiency. This breakthrough addresses the environmental concerns associated with traditional H2O2 production and offers a cleaner, more energy-efficient alternative.
Published in the prestigious journal Nature Catalysis, the research not only showcases the potential for reducing the environmental footprint of H2O2 production but also highlights the ingenuity of the NUS team in overcoming long-standing challenges in the field of catalysis.
Aside from the groundbreaking study from the National University of Singapore (NUS), other sustainable methods for hydrogen peroxide are being explored by researchers and industries. These innovative methods aim to reduce energy consumption, minimize hazardous waste production, and utilize safer, more readily available resources. Here’s a look at a few promising avenues:
- Direct Synthesis Process: This method involves producing hydrogen peroxide directly from hydrogen and oxygen, potentially in the presence of a catalyst. The attractiveness of direct synthesis lies in its simplicity and the potential reduction of energy use and toxic byproducts. Advances in catalyst development could make this a viable and eco-friendly alternative on a large scale.
- Electrochemical Production: Electrochemical processes offer a fascinating way to generate hydrogen peroxide through water electrolysis, where only water and electricity are inputs. This method can be more sustainable if powered by renewable energy sources like wind or solar power, making it an appealing green production strategy.
- Biological Production: Leveraging certain enzymes or microbes that can produce hydrogen peroxide as a byproduct of their metabolic processes opens up another sustainable pathway. This bio-based approach could lead to lower energy costs and fewer chemical requirements, presenting an eco-friendlier option that harnesses nature’s capabilities.
- Photocatalysis: A cutting-edge method involving light to catalyze a reaction between water and oxygen to produce hydrogen peroxide. Photocatalysis can be particularly sustainable if it employs sunlight as the light source, combining the abundant solar energy with catalysts often made of non-toxic materials. This process reduces the need for external energy inputs and hazardous chemicals, making it an exciting area of research for green chemistry.
- Utilizing Waste Materials: Some innovative research focuses on producing hydrogen peroxide from waste materials through various chemical or biochemical methods. This provides a new source of hydrogen peroxide and helps in waste management, aligning with circular economy principles.
Conclusion
By focusing on the atomic-level design of COFs, Professor Jiang and his team have crafted an artificial photosynthesis system that could dramatically transform industries reliant on H2O2 and pave the way for future sustainable manufacturing of chemicals. This development also highlights the role of academia in driving positive change towards a greener future.
As we continue to advance towards a more sustainable future, such pioneering efforts offer hope and inspiration for a world where environmental stewardship and industrial progress go hand in hand.